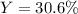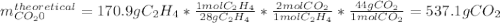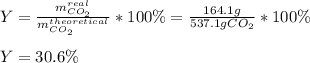Chemistry, 29.05.2020 20:02 kargarzadehsm
In an experiment, 170.9 g of C2H4 was reacted with an excess of O2, 164.1 g of CO2 is produced.
C2H4 (g) + 3 O2(g) → 2 CO2 (g) + 2 H2O (l)
What is the percent yield of this reaction?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

You know the right answer?
In an experiment, 170.9 g of C2H4 was reacted with an excess of O2, 164.1 g of CO2 is produced.
Questions

Social Studies, 26.08.2019 21:30

English, 26.08.2019 21:30

Social Studies, 26.08.2019 21:30


Mathematics, 26.08.2019 21:30



Mathematics, 26.08.2019 21:30



Biology, 26.08.2019 21:30

Biology, 26.08.2019 21:30

Arts, 26.08.2019 21:30


Social Studies, 26.08.2019 21:30

Biology, 26.08.2019 21:30

English, 26.08.2019 21:30

History, 26.08.2019 21:30


Mathematics, 26.08.2019 21:30