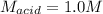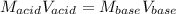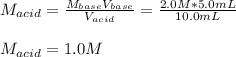Chemistry, 29.05.2020 20:01 nenelacayo07
In a titration, 5.0 ml of a 2.0 M NaOH (aq) solutions exactly neutralizes 10.0 ml of an HCL (aq) solution. what is the concentration of the HCl (aq) solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2


Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
In a titration, 5.0 ml of a 2.0 M NaOH (aq) solutions exactly neutralizes 10.0 ml of an HCL (aq) sol...
Questions