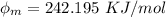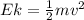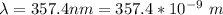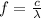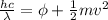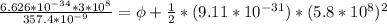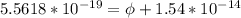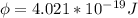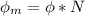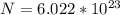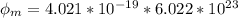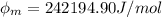Chemistry, 29.05.2020 09:57 lilyrockstarmag
In order to comply with the requirement that energy be conserved, Einstein showed in the photoelectric effect that the energy of a photon (h) absorbed by a metal is the sum of the work function (), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron: h = + Ek. When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is (in kJ/mol) of potassium?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
In order to comply with the requirement that energy be conserved, Einstein showed in the photoelectr...
Questions




















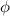 ), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron:
), the minimum energy needed to dislodge an electron from the metal's surface, and the kinetic energy (Ek) of the electron: 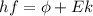 . When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is work function (in kJ/mol) of potassium?
. When light of wavelength 357.4 nm falls on the surface of potassium metal, the speed (u) of the dislodged electron is 5.8×105 m/s. What is work function (in kJ/mol) of potassium?