
Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 10:00
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3

Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3
You know the right answer?
The molecular weight of Calcium Carbonate, CaCO3, is 100.09g/mol...
Questions



Mathematics, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01


Chemistry, 16.10.2020 06:01



English, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01

Spanish, 16.10.2020 06:01

Computers and Technology, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01


Health, 16.10.2020 06:01

History, 16.10.2020 06:01

Physics, 16.10.2020 06:01

Social Studies, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01

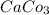 :
:

