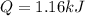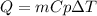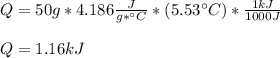Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
How much heat, in kJ, is required to raise the temperature of 50 g of water by 5.53°C? (Round to the...
Questions

Mathematics, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30

Biology, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30



Mathematics, 03.07.2019 13:30


Mathematics, 03.07.2019 13:30



Mathematics, 03.07.2019 13:30


Mathematics, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30

Mathematics, 03.07.2019 13:30