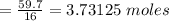Given:
CH4 + 2O2 → CO2 + 2H2O, ΔH = -890 kJ/mol
How much energy is released when 5...

Chemistry, 30.05.2020 00:03 23jaywilliams
Given:
CH4 + 2O2 → CO2 + 2H2O, ΔH = -890 kJ/mol
How much energy is released when 59.7 grams of methane (CH4) reacts with oxygen?
The combustion of 59.7 grams of methane releases kilojoules of energy

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2


Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
Questions


Mathematics, 13.10.2020 18:01

Mathematics, 13.10.2020 18:01

Chemistry, 13.10.2020 18:01

History, 13.10.2020 18:01


English, 13.10.2020 18:01


Mathematics, 13.10.2020 18:01

History, 13.10.2020 18:01

Mathematics, 13.10.2020 18:01


Biology, 13.10.2020 18:01


Mathematics, 13.10.2020 18:01

History, 13.10.2020 18:01

Mathematics, 13.10.2020 18:01

Engineering, 13.10.2020 18:01