
Chemistry, 30.05.2020 06:00 caldonia2018
Balance the equation NH3+O2--->N2+H2O given 1.37 mol of the reactant NH3 , determine the corresponding amount of O2. Answer in units of mol.?
Find the corresponding amount of N2. Answer in units of mol.
Find the corresponding amount of H2O. Answer in units of mol.
Given 3.82 mol of the product N2, find the corresponding amount of NH3. Answer in units of mol.
Find the corresponding amount of O2. Answer in units of mol.
Find the corresponding amount of N2. Answer in units of mol.
Find the corresponding amount of H2O. Answer in units of mol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
Balance the equation NH3+O2--->N2+H2O given 1.37 mol of the reactant NH3 , determine the correspo...
Questions






Mathematics, 25.01.2021 22:10

Mathematics, 25.01.2021 22:10



Mathematics, 25.01.2021 22:10

History, 25.01.2021 22:10






Mathematics, 25.01.2021 22:10

Mathematics, 25.01.2021 22:10


Mathematics, 25.01.2021 22:10

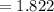 moles
moles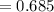 moles
moles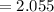 moles
moles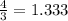 moles of Oxygems
moles of Oxygems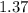 moles of NH3 shall combine with
moles of NH3 shall combine with 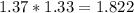 moles of oxygen (O2)
moles of oxygen (O2)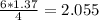 moles of water
moles of water

