

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3

Chemistry, 23.06.2019 17:00
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
What is the pH of 1.0 L of 0.20 M aqueous solution of ammonium chloride () to which 150 ml of a 0.50...
Questions



History, 08.10.2019 06:30




Spanish, 08.10.2019 06:30


Mathematics, 08.10.2019 06:30



English, 08.10.2019 06:30

English, 08.10.2019 06:30

Geography, 08.10.2019 06:30

Mathematics, 08.10.2019 06:30

Mathematics, 08.10.2019 06:30

History, 08.10.2019 06:30

Mathematics, 08.10.2019 06:30


English, 08.10.2019 06:30

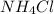 ) to which 150 ml of a 0.50 M aqueous NaOH solution is added? Assuming that
) to which 150 ml of a 0.50 M aqueous NaOH solution is added? Assuming that 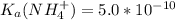 in this exercise.
in this exercise.

