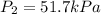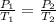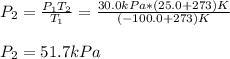Chemistry, 30.05.2020 19:02 hectorav6619
The temperature of a sample of gas in a steel
tank at 30.0 kPa is increased from -100.0°C to
25.0 °C. What is the final pressure inside the
tank?
A. 5.17 kPa
B. 51.7 kPa
C. 517 kPa
D. 5170 kPa

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
The temperature of a sample of gas in a steel
tank at 30.0 kPa is increased from -100.0°...
tank at 30.0 kPa is increased from -100.0°...
Questions


Social Studies, 13.01.2020 01:31

Mathematics, 13.01.2020 01:31


Chemistry, 13.01.2020 01:31


Mathematics, 13.01.2020 01:31

Mathematics, 13.01.2020 01:31

Mathematics, 13.01.2020 01:31



Physics, 13.01.2020 01:31


Mathematics, 13.01.2020 01:31



Advanced Placement (AP), 13.01.2020 01:31