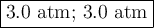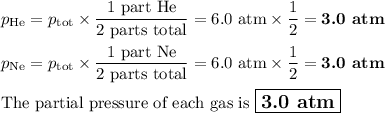Chemistry, 30.05.2020 08:59 mayamabjishovrvq9
A gas mixture contains an equal number of moles of He and Ne
The total pressure of the mixture is 6.0 atm
What is the partial pressure of He?
What is the partial pressure of Ne?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
A gas mixture contains an equal number of moles of He and Ne
The total pressure of the mixture...
The total pressure of the mixture...
Questions







Computers and Technology, 30.09.2021 23:00


Mathematics, 30.09.2021 23:00



Mathematics, 30.09.2021 23:00

Spanish, 30.09.2021 23:00





Geography, 30.09.2021 23:00

Health, 30.09.2021 23:00