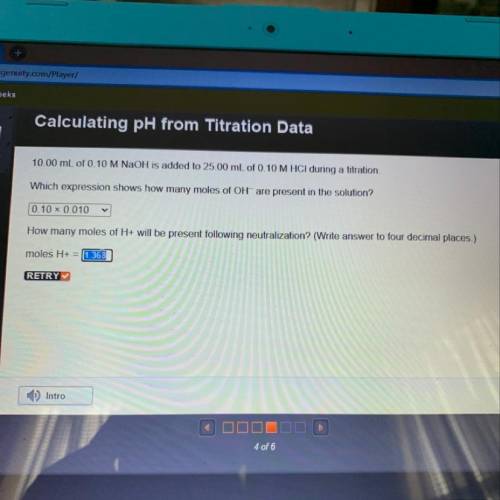
10.00 mL of 0.10 M NaOH is added to 25.00 mL of 0.10 M HCl during a titration,
Which expressio...

10.00 mL of 0.10 M NaOH is added to 25.00 mL of 0.10 M HCl during a titration,
Which expression shows how many moles of OH- are present in the solution?
How many moles of H+ will be present following neutralization? (Write answer to four decimal places.)
moles H+ =

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
Questions


Mathematics, 17.06.2021 06:20

Mathematics, 17.06.2021 06:20


Mathematics, 17.06.2021 06:20


History, 17.06.2021 06:20

Physics, 17.06.2021 06:20

History, 17.06.2021 06:20

Mathematics, 17.06.2021 06:20

Mathematics, 17.06.2021 06:20

Mathematics, 17.06.2021 06:20




English, 17.06.2021 06:20

Biology, 17.06.2021 06:20



Mathematics, 17.06.2021 06:20



