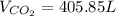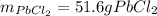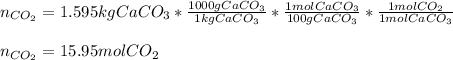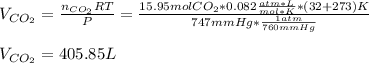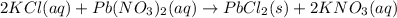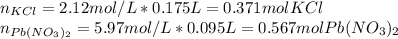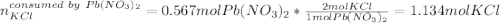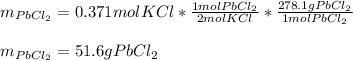Chemistry, 30.05.2020 22:59 tiwaribianca475
1) How many liters of carbon dioxide gas will be produced at 32°C and 747 mmHg if 1.595 kg of calcium carbonate decomposes? (calcium oxide is the other product)
2) If 175.0mL of a 2.12M potassium chloride solution is mixed with 95.0mL of a 5.97M lead (II) nitrate solution, how many grams of precipitate will be produced in this reaction?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
1) How many liters of carbon dioxide gas will be produced at 32°C and 747 mmHg if 1.595 kg of calciu...
Questions

History, 26.07.2019 09:30

History, 26.07.2019 09:30



Mathematics, 26.07.2019 09:30


History, 26.07.2019 09:30

Mathematics, 26.07.2019 09:30



History, 26.07.2019 09:30


Mathematics, 26.07.2019 09:30