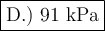Chemistry, 31.05.2020 05:00 jenadkrson62
There are 0.55 moles of carbon dioxide gas in a 15.0 L container. This container is at a temperature of 300 K. What is the pressure of the gas inside the container? Use 8.31 L*kPa/mol*K for the gas constant.
A.)760 mm Hg\
B.) 271 kPa
C.) 2 atm
D.) 91.4 kPa

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
There are 0.55 moles of carbon dioxide gas in a 15.0 L container. This container is at a temperature...
Questions




Mathematics, 06.03.2020 08:13

Mathematics, 06.03.2020 08:13



Computers and Technology, 06.03.2020 08:13




Biology, 06.03.2020 08:14

Biology, 06.03.2020 08:14