

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2


Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1
You know the right answer?
Calculate the molar mass of each if the following
a. hydrogen fluoride
b. ammonia
c...
a. hydrogen fluoride
b. ammonia
c...
Questions

English, 26.01.2021 23:50

Computers and Technology, 26.01.2021 23:50



Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

English, 27.01.2021 01:00

History, 27.01.2021 01:00

History, 27.01.2021 01:00



History, 27.01.2021 01:00

History, 27.01.2021 01:00


English, 27.01.2021 01:00


Physics, 27.01.2021 01:00

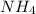 )
)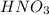 )
)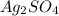 )
)

