
Chemistry, 01.06.2020 17:59 lilchannelll4125
Experiments have shown that the equilibrium constant Kp for the reaction NO(g) + NO2(g) ⇌ N2O3(g) is 0.03. A chamber with a constant total pressure initially contains NO with a partial pressure of 200 MPa, NO2 with a partial pressure of 250 MPa, and N2O3 with a partial pressure of 50 MPa. What will occur over time?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
Experiments have shown that the equilibrium constant Kp for the reaction NO(g) + NO2(g) ⇌ N2O3(g) is...
Questions


Mathematics, 06.12.2020 21:30


Mathematics, 06.12.2020 21:30


Mathematics, 06.12.2020 21:30

English, 06.12.2020 21:30

Mathematics, 06.12.2020 21:30


Biology, 06.12.2020 21:30


Mathematics, 06.12.2020 21:30


Mathematics, 06.12.2020 21:30

English, 06.12.2020 21:30

History, 06.12.2020 21:30


Mathematics, 06.12.2020 21:30


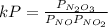 = 0.03
= 0.03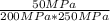 = 0.001
= 0.001

