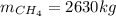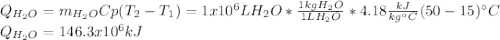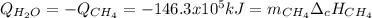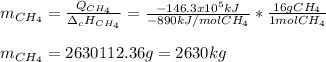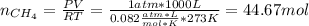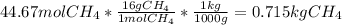En un balneario necesitan calentarse 1 millón de litros de agua anuales, subiendo la temperatura desde 15 ºC a 50 ºC y para ello utilizan calderas de gas natural (CH4), siendo la entalpía de combustión del metano de – 890 KJ/mol. Calcula:
a) El consumo anual de gas natural. Sol: 2630 Kg
b) El coste económico, anual, si el precio del m3, en condiciones normales, de gas natural es de 0,45 €. Datos Ce (H2O) = 4,18 KJ/Kg·K
Sol: 1657 €.

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

You know the right answer?
En un balneario necesitan calentarse 1 millón de litros de agua anuales, subiendo la temperatura des...
Questions



Geography, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00


Mathematics, 12.03.2021 01:00

History, 12.03.2021 01:00



Mathematics, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00

English, 12.03.2021 01:00




Mathematics, 12.03.2021 01:00


Mathematics, 12.03.2021 01:00

English, 12.03.2021 01:00