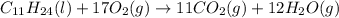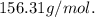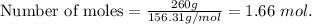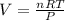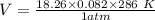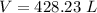Combustion of hydrocarbons such as undecane (C_11H_24) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.
1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid undecane into gaseous carbon dioxide and gaseous water.
2. Suppose 0.260 kg of undecane are burned in air at a pressure of exactly 1 atm and a temperature of 13.0°C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
Combustion of hydrocarbons such as undecane (C_11H_24) produces carbon dioxide, a "greenhouse gas."...
Questions



Mathematics, 07.06.2021 19:40




Mathematics, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40

Chemistry, 07.06.2021 19:40



Mathematics, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40


History, 07.06.2021 19:40

Mathematics, 07.06.2021 19:40


Mathematics, 07.06.2021 19:40