N2(g) + 3H2(g) <-> 2NH3(g) + energy

Chemistry, 02.06.2020 19:58 roxymiller3942
Given the equation for a system at equilibrium:
N2(g) + 3H2(g) <-> 2NH3(g) + energy
If only the concentration of N2(g) is increased, the concentration of
1. NH3(g) increases
2. NH3(g) remains the same
3. H2(g) increases
4. H2(g) remains the same

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Given the equation for a system at equilibrium:
N2(g) + 3H2(g) <-> 2NH3(g) + energy
N2(g) + 3H2(g) <-> 2NH3(g) + energy
Questions

Mathematics, 26.06.2020 22:01






Mathematics, 26.06.2020 22:01


Spanish, 26.06.2020 22:01


Mathematics, 26.06.2020 22:01


Mathematics, 26.06.2020 22:01


Mathematics, 26.06.2020 22:01



Mathematics, 26.06.2020 22:01

Mathematics, 26.06.2020 22:01

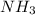 increases.
increases.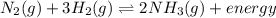
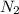 is increased , according to the Le-Chatlier's principle, the reaction has to shift to right or forward direction. In order to do that the concentration of products has to increase.
is increased , according to the Le-Chatlier's principle, the reaction has to shift to right or forward direction. In order to do that the concentration of products has to increase.

