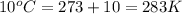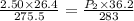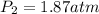7. A 26.4-ml sample of ethylene gas, C2H4, has a pres-sure of 2.50 atm at 2.5°C. If the
volume...

Chemistry, 03.06.2020 07:57 Crtive6538
7. A 26.4-ml sample of ethylene gas, C2H4, has a pres-sure of 2.50 atm at 2.5°C. If the
volume is increased to 36.2 mL and the temperature is raised to 10°C, what is the
new pressure. (Hint: Three variables have been given so what equation will you
use?)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1
You know the right answer?
Questions



English, 10.06.2021 18:30

Mathematics, 10.06.2021 18:30



History, 10.06.2021 18:30


Computers and Technology, 10.06.2021 18:30

Mathematics, 10.06.2021 18:30

Health, 10.06.2021 18:30


Mathematics, 10.06.2021 18:30



English, 10.06.2021 18:30


Biology, 10.06.2021 18:30

Chemistry, 10.06.2021 18:30

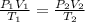
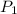 = initial pressure of gas = 2.50 atm
= initial pressure of gas = 2.50 atm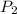 = final pressure of gas = ?
= final pressure of gas = ?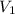 = initial volume of gas = 26.4 ml
= initial volume of gas = 26.4 ml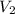 = final volume of gas = 36.2 ml
= final volume of gas = 36.2 ml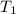 = initial temperature of gas =
= initial temperature of gas = 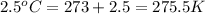
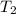 = final temperature of gas =
= final temperature of gas =