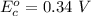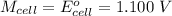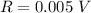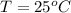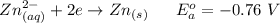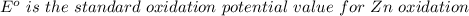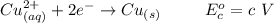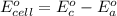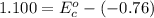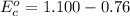Chemistry, 04.06.2020 13:20 saggin2454
Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu, and the known half life potential for Zn^2/Zn calculate the reduction potential for Cu^2+/Cu and enter value below.
The information received for this problem were the values obtained during an online lab:
Cu xM cell voltage:1.100 V
Range: 0.005 V
Temp: 25 degrees C

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu,...
Questions


Mathematics, 21.09.2019 19:00


Biology, 21.09.2019 19:00

Biology, 21.09.2019 19:00

Mathematics, 21.09.2019 19:00

English, 21.09.2019 19:00


Health, 21.09.2019 19:00

Mathematics, 21.09.2019 19:00

History, 21.09.2019 19:00

Mathematics, 21.09.2019 19:00

Mathematics, 21.09.2019 19:00

Mathematics, 21.09.2019 19:00



Mathematics, 21.09.2019 19:00



Mathematics, 21.09.2019 19:00

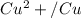 is
is