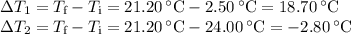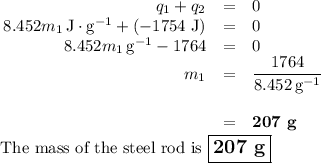CHEM EXPERT NEEDED***
A volume of 150.0 mL of H2O is initially at 24.00 °C. A chilled steel rod at 2.50 °C is placed in the water and the final temperature of the system is 21.20 °C.
Specific heat of water = 4.184 J/(g⋅∘C) and the specific heat of steel = 0.452 J/(g⋅∘C)
Write the equation and calculate the mass of the rod.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
CHEM EXPERT NEEDED***
A volume of 150.0 mL of H2O is initially at 24.00 °C. A chilled steel rod at...
Questions

Mathematics, 15.01.2020 20:31






Mathematics, 15.01.2020 20:31



Mathematics, 15.01.2020 20:31


English, 15.01.2020 20:31

Mathematics, 15.01.2020 20:31


Mathematics, 15.01.2020 20:31

Computers and Technology, 15.01.2020 20:31


Health, 15.01.2020 20:31


History, 15.01.2020 20:31