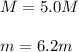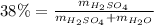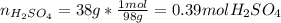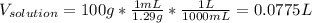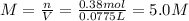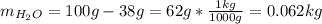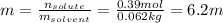Chemistry, 05.06.2020 08:59 msjsnell29
Automobile battery acid is 38% H2SO4 and has a destiny of 1.29g/ml. Calculate the molality and the molarity of this solution.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
Automobile battery acid is 38% H2SO4 and has a destiny of 1.29g/ml. Calculate the molality and the m...
Questions

English, 07.06.2021 17:20




Mathematics, 07.06.2021 17:20





Mathematics, 07.06.2021 17:20


Mathematics, 07.06.2021 17:20




Mathematics, 07.06.2021 17:20

English, 07.06.2021 17:20