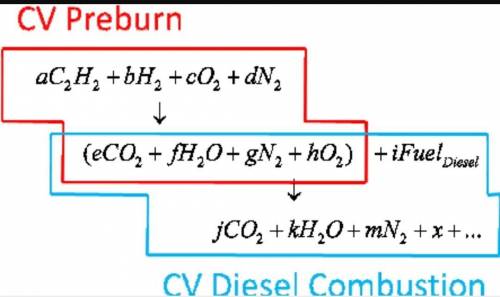
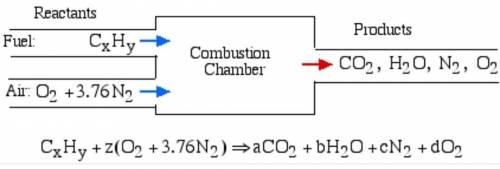
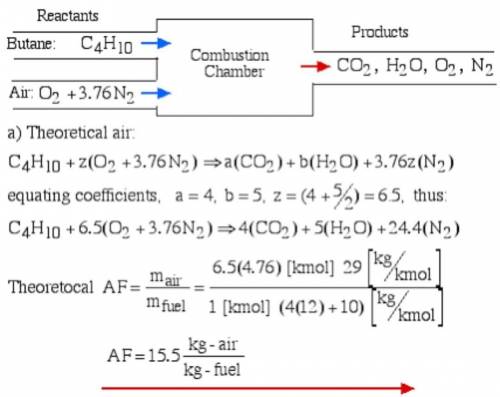
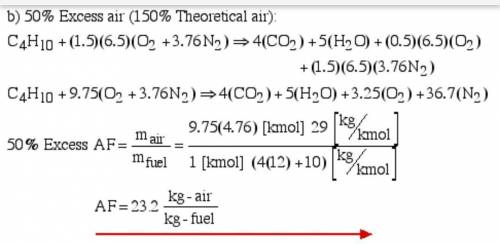
6.) A hydrocarbon molecule of
formula CxHy is completely
burnt in excess oxygen. The
comb...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
Questions

English, 10.09.2020 01:01

English, 10.09.2020 01:01

English, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01





Physics, 10.09.2020 01:01


Social Studies, 10.09.2020 01:01


Mathematics, 10.09.2020 01:01






Mathematics, 10.09.2020 01:01