
Chemistry, 06.06.2020 00:03 yuppcami9929
Br2(g) + 3 F2(g) ⇄ 2 BrF3(g) Kp = 5.4 × 108 0.30 atm of Br2 and 0.60 atm of F2 are placed in a 3.0 L container and the system is allowed to reach equilibrium. Calculate the pressureof Br2 at equilibrium.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Br2(g) + 3 F2(g) ⇄ 2 BrF3(g) Kp = 5.4 × 108 0.30 atm of Br2 and 0.60 atm of F2 are placed in a 3.0 L...
Questions



Computers and Technology, 07.05.2020 05:57


Computers and Technology, 07.05.2020 05:57


Mathematics, 07.05.2020 05:57


History, 07.05.2020 05:57


Mathematics, 07.05.2020 05:57

Mathematics, 07.05.2020 05:57




Mathematics, 07.05.2020 05:57




Social Studies, 07.05.2020 05:57

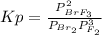 = 5.4x10⁸
= 5.4x10⁸

