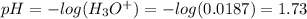A 1-liter solution contains 0.494 M hydrofluoric acid and 0.371 M potassium fluoride. Addition of 0.408 moles of hydrochloric acid will: (Assume that the volume does not change upon the addition of hydrochloric acid.)
a. Raise the pH slightly
b. Lower the pH slightly
c. Raise the pH by several units
d. Lower the pH by several units
e. Not change the pH
f. Exceed the buffer capacity

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
A 1-liter solution contains 0.494 M hydrofluoric acid and 0.371 M potassium fluoride. Addition of 0....
Questions

History, 30.10.2020 02:40

Mathematics, 30.10.2020 02:40

Mathematics, 30.10.2020 02:40

Geography, 30.10.2020 02:40

Chemistry, 30.10.2020 02:40

Geography, 30.10.2020 02:40

Social Studies, 30.10.2020 02:40


Mathematics, 30.10.2020 02:40


Biology, 30.10.2020 02:40

Social Studies, 30.10.2020 02:40




Chemistry, 30.10.2020 02:40

Mathematics, 30.10.2020 02:40


History, 30.10.2020 02:40

History, 30.10.2020 02:40

![pH = pKa + log(\frac{[KF]}{[HF]})](/tpl/images/0677/3728/a79c6.png)
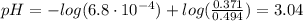
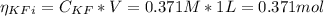
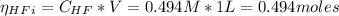
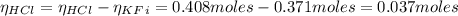
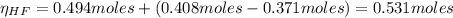
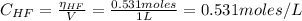
![Ka = \frac{[H_{3}O^{+}][F^{-}]}{[HF]}](/tpl/images/0677/3728/2de73.png)
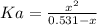 (2)
(2)