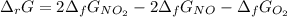(A) - 9.3 kcal

Chemistry, 06.06.2020 02:57 glowbaby123
Calculate the free energy change for the reaction:
2 NO(g) + O2(g) → 2 NO2(g)
(A) - 9.3 kcal
(B) + 24.9 kcal
(C) + 9.3 kcal
(D) - 16.6 kcal
(E) + 16.6 kcal


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3


Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
Calculate the free energy change for the reaction:
2 NO(g) + O2(g) → 2 NO2(g)
(A) - 9.3 kcal
(A) - 9.3 kcal
Questions




Mathematics, 21.02.2020 22:02




Mathematics, 21.02.2020 22:02



English, 21.02.2020 22:02



Health, 21.02.2020 22:02






Mathematics, 21.02.2020 22:02