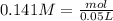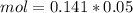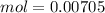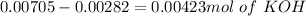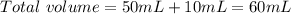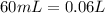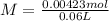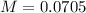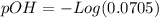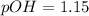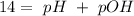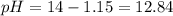Chemistry, 06.06.2020 03:59 genyjoannerubiera
A 50.0 mL solution of 0.141 M KOH is titrated with 0.282 M HCl . Calculate the pH of the solution after the addition of each of the given amounts of HCl .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
A 50.0 mL solution of 0.141 M KOH is titrated with 0.282 M HCl . Calculate the pH of the solution af...
Questions


Arts, 15.06.2021 03:00

Mathematics, 15.06.2021 03:00

Mathematics, 15.06.2021 03:00

Mathematics, 15.06.2021 03:00



Social Studies, 15.06.2021 03:00

Mathematics, 15.06.2021 03:00

Mathematics, 15.06.2021 03:00

Mathematics, 15.06.2021 03:00

English, 15.06.2021 03:00


English, 15.06.2021 03:00

Mathematics, 15.06.2021 03:00


Mathematics, 15.06.2021 03:00



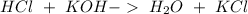
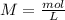
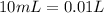 and we have the concentration of the HCl
and we have the concentration of the HCl 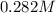 , when we plug the values into the equation we got:
, when we plug the values into the equation we got: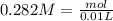
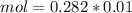
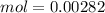
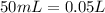 and
and 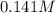 ).
).