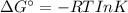Chemistry, 06.06.2020 03:57 cooljazzy1234
43. Calculate the equilibrium constant at the temperature given. (a) O2 (g) + 2F2 (g) ⟶ 2F2 O(g) (T = 100 °C) (b) I2 (s) + Br2 (l) ⟶ 2IBr(g) (T = 0.0 °C) (c) 2LiOH(s) + CO2 (g) ⟶ Li2CO3 (s) + H2 O(g) (T = 575 °C) (d) N2 O3 (g) ⟶ NO(g) + NO2 (g) (T = −10.0 °C) (e) SnCl4 (l) ⟶ SnCl4 (g) (T = 200 °C)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 23.06.2019 08:00
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3
You know the right answer?
43. Calculate the equilibrium constant at the temperature given. (a) O2 (g) + 2F2 (g) ⟶ 2F2 O(g) (T...
Questions










History, 24.07.2019 14:30

Mathematics, 24.07.2019 14:30


History, 24.07.2019 14:30


Social Studies, 24.07.2019 14:30

Social Studies, 24.07.2019 14:30

History, 24.07.2019 14:30


History, 24.07.2019 14:30

![\Delta G_f[F_2O]=41.9kJ/mol =41900J/mol](/tpl/images/0678/2670/ad240.png)
![\Delta G_f[O_2]=0\\\\ \Delta G_f[F_2]=0](/tpl/images/0678/2670/20ae9.png)
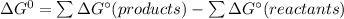
![\Delta G^\circ = [2 \times 41900]-0\\\\=83800J/mol](/tpl/images/0678/2670/d8ac8.png)