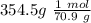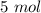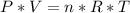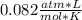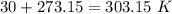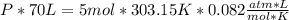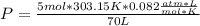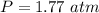Chemistry, 06.06.2020 04:01 khenalilovespandas
354.5 g of chlorine gas (MW = 70.9 g/mol) is held in a vessel with a fixed volume of 70. L.
What is the pressure of the gas in atmospheres if it's temperature is 30.0°C?
___ atm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
354.5 g of chlorine gas (MW = 70.9 g/mol) is held in a vessel with a fixed volume of 70. L.
What is...
Questions


Mathematics, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10

Social Studies, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10


Mathematics, 19.02.2021 20:10


Biology, 19.02.2021 20:10


History, 19.02.2021 20:10

History, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10



Health, 19.02.2021 20:10