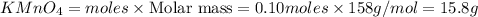Chemistry, 06.06.2020 05:01 kestegag7162
2KMnO4= K2MnO4+ MnO2+O2 how many grams of KMnO4 are required to produce 1.60 grams of O2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
2KMnO4= K2MnO4+ MnO2+O2 how many grams of KMnO4 are required to produce 1.60 grams of O2...
Questions




Arts, 25.02.2021 03:30



History, 25.02.2021 03:30


Mathematics, 25.02.2021 03:30

Mathematics, 25.02.2021 03:30



Computers and Technology, 25.02.2021 03:30

Mathematics, 25.02.2021 03:30


Chemistry, 25.02.2021 03:30

Chemistry, 25.02.2021 03:30



Mathematics, 25.02.2021 03:30

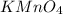 will be required to produce 1.60 grams of
will be required to produce 1.60 grams of 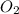
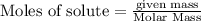
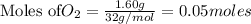
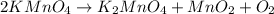
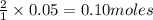 of
of