Chemistry, 05.02.2020 06:52 Thania3902
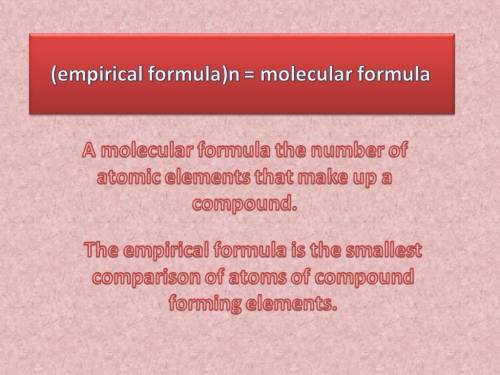
Acompound is 54.53% c, 9.15% h, and 36.32% o. what is its empirical formula?
the molar mass of the compound is 132 amu. what is its molecular formula?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
Acompound is 54.53% c, 9.15% h, and 36.32% o. what is its empirical formula?
the molar...
the molar...
Questions

History, 18.03.2021 18:20



Social Studies, 18.03.2021 18:20

Biology, 18.03.2021 18:20

Spanish, 18.03.2021 18:20



Social Studies, 18.03.2021 18:20





Biology, 18.03.2021 18:20


Chemistry, 18.03.2021 18:20


History, 18.03.2021 18:20

Advanced Placement (AP), 18.03.2021 18:20