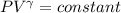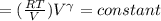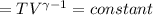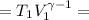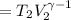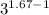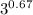Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
Calculate the final temperature of 12.0 g of Argon (considered as an ideal gas) that is expanded rev...
Questions


Mathematics, 01.04.2021 21:10

Mathematics, 01.04.2021 21:10

History, 01.04.2021 21:10

Mathematics, 01.04.2021 21:10





Physics, 01.04.2021 21:10




Mathematics, 01.04.2021 21:10


Mathematics, 01.04.2021 21:10

English, 01.04.2021 21:10

Mathematics, 01.04.2021 21:10

Biology, 01.04.2021 21:10