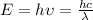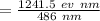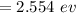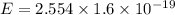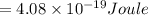Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
The teal line of the hydrogen emission spectrum has a wavelength of 486.0 nm. A hydrogen emission sp...
Questions



Mathematics, 06.11.2020 19:40

Chemistry, 06.11.2020 19:40

Biology, 06.11.2020 19:40


History, 06.11.2020 19:40




Law, 06.11.2020 19:40

English, 06.11.2020 19:40


Mathematics, 06.11.2020 19:40

History, 06.11.2020 19:40

Mathematics, 06.11.2020 19:40


Chemistry, 06.11.2020 19:40