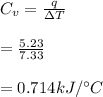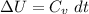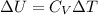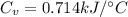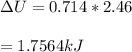Chemistry, 07.06.2020 03:58 Pookaapoo8832
Next, a chemical reaction of interest was conducted in the same constant volume calorimeter. The neutralization reaction of HCl(aq) with NaOH(aq) caused the temperature of the calorimeter to rise by 2.46 °C. What is the change in internal energy ΔU of the neutralization reaction in kJ?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Next, a chemical reaction of interest was conducted in the same constant volume calorimeter. The neu...
Questions

Social Studies, 23.08.2020 02:01

History, 23.08.2020 02:01

History, 23.08.2020 02:01

Mathematics, 23.08.2020 02:01



Physics, 23.08.2020 02:01



English, 23.08.2020 02:01

Health, 23.08.2020 02:01

Computers and Technology, 23.08.2020 02:01

Chemistry, 23.08.2020 02:01

Physics, 23.08.2020 02:01

Biology, 23.08.2020 02:01

Physics, 23.08.2020 02:01


English, 23.08.2020 02:01

Biology, 23.08.2020 02:01