
Chemistry, 07.06.2020 04:57 robert7248
Calculate the standard enthalpy of formation of NOCl(g) at 25 ºC, knowing that the standard enthalpy of formation of NO(g) at that temperature is 90.25 kJ / mol, and that the standard molar enthalpy of the reaction 2 NOCl(g) → 2 NO(g) + Cl2(g) is 75.5 kJ / mol at the same temperature.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
Calculate the standard enthalpy of formation of NOCl(g) at 25 ºC, knowing that the standard enthalpy...
Questions


Mathematics, 18.03.2021 01:40

Biology, 18.03.2021 01:40

Social Studies, 18.03.2021 01:40

Health, 18.03.2021 01:40




English, 18.03.2021 01:40




English, 18.03.2021 01:40

History, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40



Mathematics, 18.03.2021 01:40

Social Studies, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40

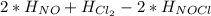
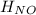 = 90.25 kJ/mol
= 90.25 kJ/mol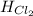 = 0 (For the formation of one mole of a pure element the heat of formation is 0, in this caseyou have as a pure compound the chlorine Cl₂)
= 0 (For the formation of one mole of a pure element the heat of formation is 0, in this caseyou have as a pure compound the chlorine Cl₂)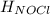 =?
=?

