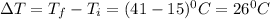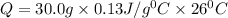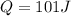Chemistry, 07.06.2020 04:58 Cannon2793
If the specific heat of gold is 0.13 J/gC, what is the amount of energy (heat) required to raise 30.0g of gold from 15 degrees Celsius to 41 degrees Celsius?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

You know the right answer?
If the specific heat of gold is 0.13 J/gC, what is the amount of energy (heat) required to raise 30....
Questions




History, 13.02.2020 05:13



Mathematics, 13.02.2020 05:13



Mathematics, 13.02.2020 05:13

Mathematics, 13.02.2020 05:13



Mathematics, 13.02.2020 05:13

Mathematics, 13.02.2020 05:13

History, 13.02.2020 05:13




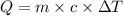
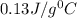
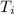 = 15°C
= 15°C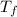 = 41°C
= 41°C