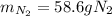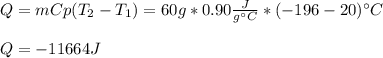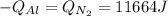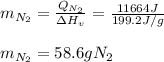Chemistry, 07.06.2020 04:58 isabella4141
A 60 g piece of aluminum at 20°C is cooled to -196°C by placing it in a large container of liquid nitrogen at that temperature. How much nitrogen is vaporized? (Assume that the specific heat of aluminum is constant and is equal to 0.90 kJ/kg·K and that the vaporized nitrogen's temperature does not change.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
A 60 g piece of aluminum at 20°C is cooled to -196°C by placing it in a large container of liquid ni...
Questions

Mathematics, 27.12.2020 07:30

Arts, 27.12.2020 07:30

Physics, 27.12.2020 07:30


Mathematics, 27.12.2020 07:30


World Languages, 27.12.2020 07:30

History, 27.12.2020 07:30

Mathematics, 27.12.2020 07:30

Health, 27.12.2020 07:30


Physics, 27.12.2020 07:30

History, 27.12.2020 07:40

Mathematics, 27.12.2020 07:40


Health, 27.12.2020 07:40


English, 27.12.2020 07:40


Physics, 27.12.2020 07:40