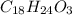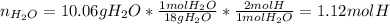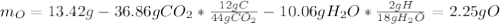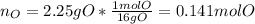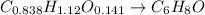Chemistry, 07.06.2020 05:00 calebwoodall6477
Combustion analysis of a 13.42-g sample of an unknown organic compound (which contains only carbon, hydrogen, and oxygen) produced 36.86 g CO2 and 10.06 g H2O. The molar mass of the compound is 288.38 g/mol . Part A Find the molecular formula of the unknown compound. Express your answer as a chemical formula.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1


Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
Combustion analysis of a 13.42-g sample of an unknown organic compound (which contains only carbon,...
Questions

Biology, 01.04.2020 21:56

Biology, 01.04.2020 21:56



Mathematics, 01.04.2020 21:56

Mathematics, 01.04.2020 21:56

Mathematics, 01.04.2020 21:56

Arts, 01.04.2020 21:56


Chemistry, 01.04.2020 21:56




Mathematics, 01.04.2020 21:56


Mathematics, 01.04.2020 21:56




English, 01.04.2020 21:56