
Chemistry, 07.06.2020 23:57 shelbysargent11
In the reaction A + B → C, the initial concentration of A is 0.054 M. After 1.5 minutes, [A] = 0.032 M. Calculate the rate of the reaction in M/sec.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
In the reaction A + B → C, the initial concentration of A is 0.054 M. After 1.5 minutes, [A] = 0.032...
Questions

Biology, 13.03.2021 01:00

Social Studies, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00


Social Studies, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Arts, 13.03.2021 01:00

Social Studies, 13.03.2021 01:00


Biology, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00



Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

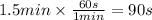
![r=-\frac{\Delta [A]}{t} =-\frac{[A]-[A]_0}{t} =-\frac{0.032M-0.054M}{90s} =2.4 \times 10^{-4} M/s](/tpl/images/0679/7433/dace5.png)


