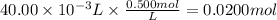Hydrobromic acid solution of unknown concentration is titrated with a 0.500M LiOH solution.
20.00mL of the acid are poured into an Erlenmeyer flask.
40.00mL of the base solution is required to reach the equivalence point.
What is the molarity of the Hydrobromic acid solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2
You know the right answer?
Hydrobromic acid solution of unknown concentration is titrated with a 0.500M LiOH solution.
20.00mL...
Questions

Business, 04.12.2020 07:20




English, 04.12.2020 07:20

Spanish, 04.12.2020 07:20

Mathematics, 04.12.2020 07:20

History, 04.12.2020 07:20





Biology, 04.12.2020 07:20



Mathematics, 04.12.2020 07:20

Mathematics, 04.12.2020 07:20

Social Studies, 04.12.2020 07:20

Chemistry, 04.12.2020 07:20

History, 04.12.2020 07:20