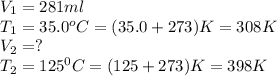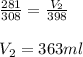Chemistry, 08.06.2020 09:57 brucewayne8499
Carbon dioxide gas at a temperature of 35.0 C and a volume of 281mL. Determine the volume of the gas when the temperature of the gas is raised to 125 C if the pressure remains constant.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

You know the right answer?
Carbon dioxide gas at a temperature of 35.0 C and a volume of 281mL. Determine the volume of the gas...
Questions

Mathematics, 12.02.2022 23:30

Computers and Technology, 12.02.2022 23:30

Mathematics, 12.02.2022 23:40

Chemistry, 12.02.2022 23:40


Mathematics, 12.02.2022 23:40

Mathematics, 12.02.2022 23:40



Biology, 12.02.2022 23:40


Mathematics, 12.02.2022 23:40

Mathematics, 12.02.2022 23:40



Chemistry, 12.02.2022 23:40




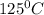 is 363 ml
is 363 ml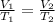
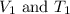 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.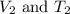 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.