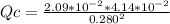Consider the following reaction where Kc = 1.80×10-2 at 698 K:
2HI(g) → H2(g) + I2(g)
A reaction mixture was found to contain 0.280 moles of HI (g), 2.09×10^-2 moles of H2 (g), and 4.14×10^-2 moles of I2 (g), in a 1.00 liter container.
Required:
a. Is the reaction at equilibrium?
b. What direction must it run in order to reach equilibrium?
c. The reaction
1. must run in the forward direction to reach equilibrium.
2. must run in the reverse direction to reach equilibrium.
3. is at equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
Consider the following reaction where Kc = 1.80×10-2 at 698 K:
2HI(g) → H2(g) + I2(g)
...
...
Questions

Mathematics, 21.10.2020 23:01



English, 21.10.2020 23:01







Mathematics, 21.10.2020 23:01


Mathematics, 21.10.2020 23:01



English, 21.10.2020 23:01


Mathematics, 21.10.2020 23:01


Mathematics, 21.10.2020 23:01

![Qc=\frac{[C]^{c}*[D]^{d} } {[A]^{a}*[B]^{b}}](/tpl/images/0680/9750/5af8a.png)
![Qc=\frac{[H_{2} ]*[I_{2} ] } {[HI]^{2}}](/tpl/images/0680/9750/8091f.png)
![[H_{2} ]=\frac{2.09*10^{-2} moles}{1 Liter}](/tpl/images/0680/9750/734fa.png) =2.09*10⁻²
=2.09*10⁻² 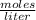
![[I_{2} ]=\frac{4.14*10^{-2} moles}{1 Liter}](/tpl/images/0680/9750/92c8b.png) =4.14*10⁻²
=4.14*10⁻² ![[I_{2} ]=\frac{0.280 moles}{1 Liter}](/tpl/images/0680/9750/e5237.png) = 0.280
= 0.280