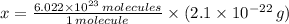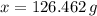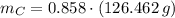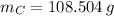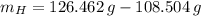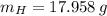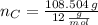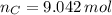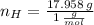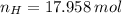Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
Un hidrocarburo tiene como composición en masa: C= 85.8% ; H= 14.2% . Como dato nos brindan que una...
Questions



Mathematics, 12.02.2021 18:10

Mathematics, 12.02.2021 18:10


Mathematics, 12.02.2021 18:10

Mathematics, 12.02.2021 18:10


English, 12.02.2021 18:10


Mathematics, 12.02.2021 18:10


Mathematics, 12.02.2021 18:10

English, 12.02.2021 18:10

Social Studies, 12.02.2021 18:10

Mathematics, 12.02.2021 18:10

Chemistry, 12.02.2021 18:10

Mathematics, 12.02.2021 18:10

History, 12.02.2021 18:10

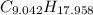
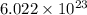 moléculas. La masa de un mol se determina mediante regla de tres simple:
moléculas. La masa de un mol se determina mediante regla de tres simple: