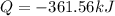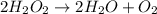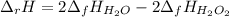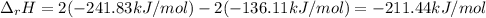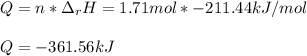Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
The decomposition of hydrogen peroxide, H2O2, has been used to provide thrust in the control jets of...
Questions


Engineering, 17.04.2021 14:00





Mathematics, 17.04.2021 14:00


Social Studies, 17.04.2021 14:00

English, 17.04.2021 14:00


Computers and Technology, 17.04.2021 14:00

Chemistry, 17.04.2021 14:00

English, 17.04.2021 14:00

Chemistry, 17.04.2021 14:00

English, 17.04.2021 14:00

English, 17.04.2021 14:00