
Chemistry, 10.06.2020 18:57 gretchcampbell
Acetic acid and ethanol react to form ethyl acetate and water. If 94.0 mmol of C2H5CO2CH3 are removed from a flask containing a mixture of HCH3CO2, C2H5OH, C2H5CO2CH3 and H2O at equilibrium, then following questions are to be answered. 1. What is the rate of the reverse reaction before any C2H5CO2CH3 has been removed from the flask?2. What is the rate of the reverse reaction just after the C2H5CO2CH3 has been removed from the flask?3. What is the rate of the reverse reaction when the system has again achieved equilibrium?4. How much less C2H5CO2CH3 is in the flask when the system has again reached equilibrium?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Acetic acid and ethanol react to form ethyl acetate and water. If 94.0 mmol of C2H5CO2CH3 are remove...
Questions

History, 24.06.2019 04:00


Mathematics, 24.06.2019 04:00




Chemistry, 24.06.2019 04:00

Spanish, 24.06.2019 04:00


Physics, 24.06.2019 04:00


Social Studies, 24.06.2019 04:00

Mathematics, 24.06.2019 04:00







Spanish, 24.06.2019 04:00

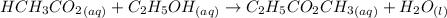
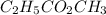 are removed from a flask; Then:
are removed from a flask; Then:

