Chemistry, 10.06.2020 20:57 AldoRaine8074
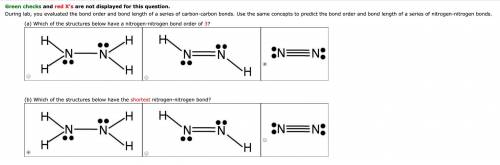
During lab, you evaluated the bond order and bond length of a series of carbon-carbon bonds. Use the same concepts to predict the bond order and bond length of a series of nitrogen-nitrogen bonds.(a) Which of the structures below have a nitrogen-nitrogen bond order of 3?(b) Which of the structures below have the shortest nitrogen-nitrogen bond?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
During lab, you evaluated the bond order and bond length of a series of carbon-carbon bonds. Use the...
Questions


Physics, 29.05.2020 19:58

History, 29.05.2020 19:58


Mathematics, 29.05.2020 19:58

Mathematics, 29.05.2020 19:58


Health, 29.05.2020 19:59







Mathematics, 29.05.2020 19:59

Mathematics, 29.05.2020 19:59

Mathematics, 29.05.2020 19:59

Mathematics, 29.05.2020 19:59