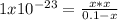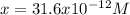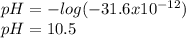Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
consider an exceptionally weak acid, HA, with Ka= 1 x 10-20. you make 0.1M solution of the salt NA....
Questions

Mathematics, 27.09.2021 18:20

Biology, 27.09.2021 18:20

Mathematics, 27.09.2021 18:20




History, 27.09.2021 18:20

Social Studies, 27.09.2021 18:20

Social Studies, 27.09.2021 18:20

Computers and Technology, 27.09.2021 18:20


English, 27.09.2021 18:20

Mathematics, 27.09.2021 18:20



English, 27.09.2021 18:20

History, 27.09.2021 18:20

Mathematics, 27.09.2021 18:20

Mathematics, 27.09.2021 18:20

History, 27.09.2021 18:20

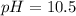
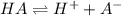
![Ka=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0682/3092/39962.png)
 due to the reaction extent is:
due to the reaction extent is: