Chemistry, 11.06.2020 01:57 dellian5355
How many moles of gas are contained in 22.41 liters at 101.325 kPa and 0ᴼC? (Note: use Ideal Gas Law, PV = nRT) a 2.5 mole b 1.5 mole c 1.0 mole d 2.0 mole

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

You know the right answer?
How many moles of gas are contained in 22.41 liters at 101.325 kPa and 0ᴼC? (Note: use Ideal Gas Law...
Questions


English, 18.03.2021 21:50

Social Studies, 18.03.2021 21:50

Mathematics, 18.03.2021 21:50

Mathematics, 18.03.2021 21:50


Mathematics, 18.03.2021 21:50




English, 18.03.2021 21:50

Mathematics, 18.03.2021 21:50








Mathematics, 18.03.2021 21:50

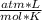 T= 0°C= 273 °K
T= 0°C= 273 °K