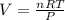Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
What does Avogadro's law say about a gas atSTP? A; 1 L of any gas contains 22.4 moles at STP B; 1 L...
Questions


Mathematics, 15.04.2021 21:00



Mathematics, 15.04.2021 21:00

Physics, 15.04.2021 21:00

Physics, 15.04.2021 21:00

French, 15.04.2021 21:00


English, 15.04.2021 21:00




Health, 15.04.2021 21:00


Mathematics, 15.04.2021 21:00

Chemistry, 15.04.2021 21:00


English, 15.04.2021 21:00

English, 15.04.2021 21:00

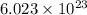 of particles.
of particles.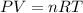
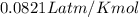
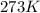 (at STP)
(at STP)