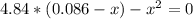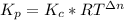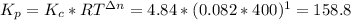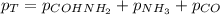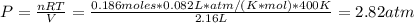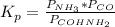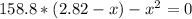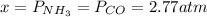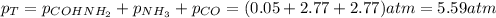Chemistry, 11.06.2020 02:57 dontworry48
Formamide decomposes at high temperature. If 0.186 mol of formamide (HCONH2) dissociates in a 2.16 L flask at 400 K, what are the concentrations of all species present at equilibrium at 400 K? (hint: calculate concentrations first) (b) What is the total pressure in the container at equilibrium?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
Formamide decomposes at high temperature. If 0.186 mol of formamide (HCONH2) dissociates in a 2.16 L...
Questions



Physics, 07.03.2020 00:42





Business, 07.03.2020 00:42


Computers and Technology, 07.03.2020 00:42


History, 07.03.2020 00:42



Mathematics, 07.03.2020 00:42

English, 07.03.2020 00:42


Health, 07.03.2020 00:43


![K_{c} = \frac{[NH_{3}][CO]}{[COHNH_{2}]} = 4.84 (400 K)](/tpl/images/0682/5560/28fef.png)
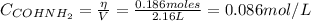
![K_{c} = \frac{[NH_{3}][CO]}{[COHNH_{2}]} = \frac{x*x}{0.086 - x}](/tpl/images/0682/5560/05a27.png)